Review Article
Oocyte Quality & its Impact on the Reproductive Outcomes of Women Undergoing Assisted Reproduction: A Review
Nidhi Srivastava, Richa Saxena
Licence:
Attribution-Non-commercial 4.0 International (CC BY-NC 4.0)This license enables reusers to distribute, remix, adapt, and build upon the material in any medium or format for noncommercial purposes only, and only so long as attribution is given to the creator
RFP Journal of Biochemistry and Biophysics 9(1):p 91-96, Jan-June 2024. | DOI: http://dx.doi.org/10.21088/rfpjbb.2582-3558.9124.4
How Cite This Article:
Saxena R, Srivastava N. Oocyte quality & its impact on the reproductive outcomes of women undergoing assisted reproduction: a review. RFP J Bio Biophy. 2024;9(1):91-6.Timeline
Abstract
The Assisted Reproductive Technology (ART) has led to technical advancements in the last few years. These techniques have greatly assisted in achieving an acceptable pregnancy rate. Pregnancy followed by delivery signifies the success of an ART treatment. The success rate of an ART treatment is hinged on various parameters of which oocytes have a primary role in fertilization, early embryo development and its subsequent implantation. Successful pregnancies are common in assisted reproductive technology clinics because of invasive and non-invasive methods used to isolate biologically competent oocytes. The process of fertilizing the embryo, early embryo growth, implanting of the fertilized embryo, and favorable pregnancy results may be predicted by morphological features like zonapellucida, the cumulus complex, first polarized body, perivitelline membrane area space, spindle formation assembly, and ooplasm. The non-invasive assessment of oocyte quality based on cumulus gene expression analysis in conjunction with morphology assessment can improve the clinical pregnancy (CPR) and live birth rates (LBR). The infertility that is linked with poor oocyte quality may be explained by a number of different processes that are not exclusive to one another. To a large extent, the success of in vitro fertilization (IVF) depends on the oocyte, which plays a critical role in defining embryonic competence. It has been suggested in research studies that the shape of oocytes may serve as a non-invasive indicator of the quality of the oocytes. The current review investigates the correlation of oocyte quality and its effect on the clinical outcomes of women undergoing regulated ovarian stimulating for an intracytoplasmic sperm injection (ICSI).
References
- 1. Bulun SE, Yilmaz BD, Sison C, Miyazaki K, Bernardi L, Liu S, et al. Endome- triosis. Endocr Rev. 2019; 40(4):1048-79doi: 10.1210/er.2018 00242Pubmed:30994890.
- 2. Asa E, TabatabaeeRM, Farrokhi A, NejatbakhshR. Relationship between meiotic spindles visualization and intracytoplasmic sperm injection outcomes in human oocytes. AnatCell Biol. 2017; 50(1):26-6doi: 10.5115/acb.2017.50.1.26.
- 3. Liu T, Liu D, Song X, Qu J, Zheng X, Li J, et al. Lipid metabolismwasassociatedwithoocyte in vitro maturation in womenwithpolycysticovariansynd romeundergoingunstimulatednaturalcycle. Front CellDevBiol. 2021;9:719173doi: 10.3389/fcell.2021.7 19173Pubmed:34540838.
- 4. Lemseffer Y, TerretME, Campillo C, Labrune E. Methods for assessingoocytequality: areview of literature. Biomedicines. 2022; 10(9):2184doi: 10.3390/biomedicines10092184Pubmed:36140285.
- 5. Nikbakht R, Mohammadjafari R, Rajabalipour M, Moghadam MT. Evaluation of oocyte quality in polycystic ovary syndrome patients undergoing ART cycles. FertilRes Pract. 2021;7(1):2doi: 10.1186/ s40738-020-00094-zPubmed:33397466.
- 6. Mikkelsen AL, Lindenberg S. Morphology of in-vitro matured oocytes: impact on fertility potential and embryo quality. HumReprod. 2001; 16(8):1714-8doi: 10.1093/humrep/16.8.1714Pubm ed:11473970.
- 7. Van Blerkom J, Henry G. Oocyte dysmorphism and aneuploidy in meiotically mature human oocytes after ovarian stimulation. Hum Reprod.1992;7(3):379-90doi: 10.1093/ oxfordjournals.humrep.a137655Pubmed:1587948.
- 8. Latif S, Saridogan E. Endometriosis, oocyte, and embryo quality. J ClinMed.2023;12(13):4186doi: 10.3390/jcm12134186Pubmed:37445220.
- 9. Guimarães RMGC, Ribeiro LM, Sasaki LP, Nakagawa HM, Cabral IO. Oocyte morphology and reproductive outcomes - case report and literature review. JBRA Assist Reprod.2021;25(3):500-7doi: 10.5935/1518-0557.20210001Pubmed:33739798.
- 10. Ozturk S. Selection of competent oocytes by morphological criteria for assisted reproductive technologies. MolReprodDev.2020;87(10):1021 36doi: 10.1002/mrd.23420Pubmed:32902927.
- 11. Malhotra N, Shah D, Pai R, Pai HD, Bankar M. Assisted reproductive technology in India: A 3 year retrospective data analysis. J Hum ReprodSci.2013;6(4):235-40doi: 10.4103/0974-1208.126286Pubmed:24672161.
- 12. Murphy MK, Hall JE, Adams JM, Lee H, Welt CK. Polycystic ovarian morphology in normal women does not predict the development of polycystic ovary syndrome. J ClinEndocrinolMetab. 2006; 91(10):3878-84doi: 10.1210/jc.2006 1085Pubmed:16882750.
- 13. Sigala J, Sifer C, Dewailly D, Robin G, Bruyneel A, Ramdane N, et al. Is polycystic ovarian morphology related to a poor oocyte quality after controlled ovarian hyperstimulation for intracytoplasmic sperm injection? Results from a prospective, comparative study. FertilSteril. 2015; 103(1):112 8doi: 10.1016/j.fertnstert.2014.09.040Pubm ed:25450303.
- 14. Lizneva D, Suturina L, Walker W, BRakta S, Gavrilova-Jordan L, Azziz R. Criteria, prevalence, and phenotypes of polycysticovarysyndrome. FertilSteril. 2016; 106(1):6-15doi: 10.1016/j.fertnste rt.2016.05.003Pubmed:27233760.
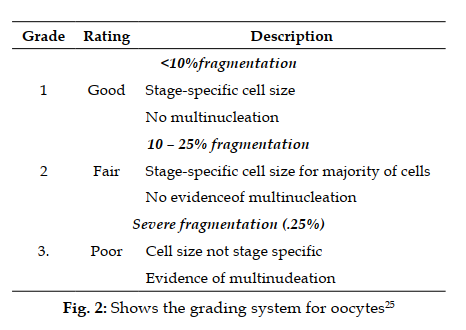
- 15. Catteau-Jonard S, Dewaily D. Pathophysiology of disturbedfolliculogenesis in PCOS. Med Reprod. 2009; 11(3):191-7 doi: 10.1684/mte.2009.0241.
- 16. Scott L. The biologicalbasis of non-invasiveStratergies for selection of humanoocytes and embryos. Hum Reprod Update. 2003; 9(3):237-49doi: 10.1093/ humupd/dmg023Pubmed:12859045.
- 17. Sayutti N, Abu MA, Ahmad MF. PCOS and role of cumulusgeneexpression in assessingoocytesquality. Front Endocrinol. 2022; 13:843867doi: 10.3389/fend o.2022.843867Pubmed:35721714.
- 18. Patrizio P, Sakkas D. From oocyte to baby: a clinical evaluation of the biological efficiency of in vitro fertilization. FertilSteril. 2009; 91(4):1061-6doi: 10.1016/j.fertnstert.2008.01.003Pubmed:18325517.
- 19. Magaton IM, Helmer A, Eisenhut M, Roumet M, Stute P, von Wolff M. Oocyte maturity, oocyte fertilization and cleavage-stage embryo morphology are better in natural compared with high-dose gonadotrophin stimulated IVF cycles. ReprodBiomed Online. 2023; 46(4):705-12doi: 10.1016/j.rbmo.2022.11.008Pubmed:36754739.
- 20. Han E, Seifer DB. Oocyte cryopreservation for medical and plannedindications: A practicalguide and overview. J ClinMed. 2023;12(10):3542doi: 10.3390/jcm12103542Pubmed:37240648.
- 21. FauserBC. Towards the global coverage of a unified registry of IVF outcomes. ReprodBiomed Online. 2019; 38(2):133-7 doi: 10.1016/j.rbmo.2018.12.001P ubmed:30593441.
- 22. Stamenov G, Parvanov D, Chaushev T, Baltadzhieva D, Iliev I, Dzhambazov B. Approaches for prediction of the implantation potential of human embryos. J BioSciBiotechnol. 2013; 2:79-88.
- 23. Lazzaroni-Tealdi E, Barad DH, Albertini DF, Yu Y, Kushnir VA, Russell H, et al. Oocyte Scoring Enhances Embryo-Scoring in Predicting Pregnancy Chances with IVF Where It Counts Most. Plos One. 2015; 10:e0143632.
- 24. Lasiene K, Lasys V, Glinskyte S, Valanciute A, Vitkus A. Relevance and methodology for the morphologicalanalysis of oocytequality in IVF and ICSI. J ReprodStem Cell Biotechnol. 2011;2(1):1 13doi: 10.1177/205891581100200102.
- 25. Halim B, Lubis HP, Novia D, Thaharuddin M. Does oval oocyte have an impact on embryo development in in vitro fertilization? JBRA AssistReprod. 2017; 21(1):15-8doi: 10.5935/1518-0557.20170005Pubm ed:28333026.
Data Sharing Statement
Funding
Author Contributions
Ethics Declaration
Acknowledgements
Conflicts of Interest
About this article
Cite this article
Saxena R, Srivastava N. Oocyte quality & its impact on the reproductive outcomes of women undergoing assisted reproduction: a review. RFP J Bio Biophy. 2024;9(1):91-6.
Licence:
Attribution-Non-commercial 4.0 International (CC BY-NC 4.0)This license enables reusers to distribute, remix, adapt, and build upon the material in any medium or format for noncommercial purposes only, and only so long as attribution is given to the creator
| Received | Accepted | Published |
|---|---|---|
| May 14, 2024 | June 17, 2024 | June 30, 2024 |
DOI: http://dx.doi.org/10.21088/rfpjbb.2582-3558.9124.4
Keywords
EmbryoPregnancyIn-vitro fertilizationEndometriosisPolycystic ovarian syndrome.PregnancyIn-vitro fertilizationEndometriosisPolycystic ovarian syndrome.Search for Similar Articles
Similar Articles
- Pharmacological and Phytochemical Insights into Strobilanthes alternata: A Multi...
- The Unseen Pillars: The Foundational Role of Biophysics and Biochemistry in Mode...
- Screen time and its Biochemical Effect on Human beings
- Endocrine Disruption by Heavy Metals: An In-Depth Analytical Review
- How Alloying Affects the Critical Temperature of Low-Temperature Superconductors...
Article Level Metrics
Last UpdatedMonday 26 January 2026, 20:22:50 (IST)
Accesses
Citations
Download citation
Article Keywords
Keyword Highlighting
Highlight selected keywords in the article text.
Timeline
| Received | May 14, 2024 |
| Accepted | June 17, 2024 |
| Published | June 30, 2024 |
licence
This license enables reusers to distribute, remix, adapt, and build upon the material in any medium or format for noncommercial purposes only, and only so long as attribution is given to the creator