Original Article
Endocrine Disruption by Heavy Metals: An In-Depth Analytical Review
Suyash Saxena, Rahul Saxena, Ajit Pal Singh, Abdullah Salim Al-Karawi, Ali Saad Kadhim
Licence:
Attribution-Non-commercial 4.0 International (CC BY-NC 4.0)This license enables reusers to distribute, remix, adapt, and build upon the material in any medium or format for noncommercial purposes only, and only so long as attribution is given to the creator.
RFP Journal of Biochemistry and Biophysics 10(2):p 51-60, July-December 2025. | DOI: 10.21088/rfpjbb.2582-3558.10225.2
How Cite This Article:
Saxena S, Saxena R, Singh AP. Endocrine disruption by heavy metals: an in-depth analytical review. RFP Jour of Bio and Biophy. 2025;10(2):51–60.Timeline
Abstract
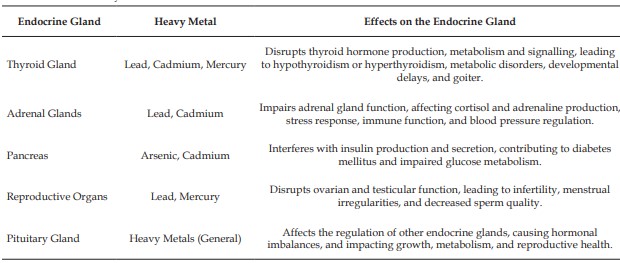
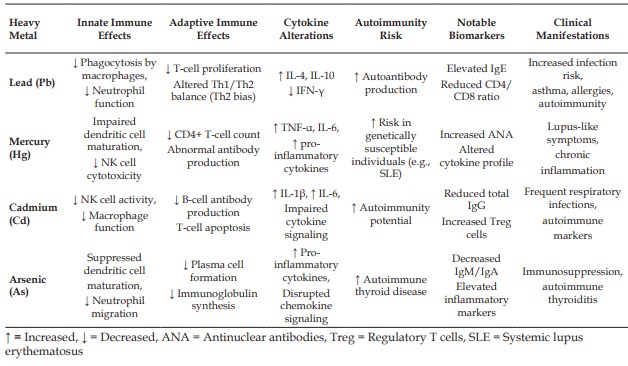
Lead, mercury, cadmium, and arsenic are well-known heavy metals that are potentially toxic and continue to impact the environment. These compounds can interfere with the endocrine system, causing drastic effects on individual health. Endocrine disruptors can cause hormonal dysfunction at the site of production, release, transport, metabolism, binding, action, or elimination leading to reproductive, developmental, neurological, and immune disorders in humans and wildlife. For example, cadmium imitates estrogen and binds to estrogen receptors, triggering pathological hormonal responses. Lead exposure has been linked to alterations in the hypothalamic-pituitary-adrenal axis, affecting stress hormone balance and cognitive performance. Mercury disrupts thyroid hormone metabolism, impairing thyroid function and child development. Heavy metals also harm the immune system. Lead and cadmium weaken both general and specific immune defences, increase susceptibility to infections, alter cytokine signalling, and raise the risk of autoimmune diseases. Mercury is associated with weakened immune responses and abnormal antibody production. These changes compromise the body’s ability to protect itself and elevate overall health risks. These metals accumulate in the food chain, posing greater threats to both human health and the ecological environment. Studying the molecular mechanisms of endocrine disruption by heavy metals is crucial for developing effective policies and protective strategies. Government and health guidelines should focus on minimizing environmental contamination, improving diagnostic tools, and raising public awareness. Coordinated efforts from governments, academia, industry, and communities are essential to safeguard present and future generations and promote a healthier world.
References
- 1. Dutta S, Gorain B, Choudhury H, Roychoudhury S, Sengupta P. Environmental and occupational exposure of metals and female reproductive health. Environmental Science and Pollution Research. 2022 Sep;29(41):62067-92.
- 2. Boskabady M, Marefati N, Farkhondeh T, Shakeri F, Farshbaf A, Boskabady MH. The effect of environmental lead exposure on human health and the contribution of inflammatory mechanisms, a review. Environment international. 2018 Nov 1;120:404-20.
- 3. Buha A, Matovic V, Antonijevic B, Bulat Z, Curcic M, Renieri EA, Tsatsakis AM, Schweitzer A, Wallace D. Overview of cadmium thyroid disrupting effects and mechanisms. International Journal of Molecular Sciences. 2018 May 17;19(5):1501.
- 4. Ohiagu FO, Chikezie PC, Ahaneku CC, Chikezie CM. Human exposure to heavy metals: toxicity mechanisms and health implications. Material Sci Eng. 2022;6(2):78-87.
- 5. Masindi V, Muedi KL. Environmental contamination by heavy metals. Heavy metals. 2018 Jun 27;10(4):115-33.
- 6. Ali H, Khan E, Ilahi I. Environmental chemistry and ecotoxicology of hazardous heavy metals: environmental persistence, toxicity, and bioaccumulation. Journal of chemistry. 2019;2019(1):6730305.
- 7. Skerfving S, Löfmark L, Lundh T, Mikoczy Z, Strömberg U. Late effects of low blood lead concentrations in children on school performance and cognitive functions. Neurotoxicology. 2015 Jul 1;49:114-20.
- 8. Ali W, Ma Y, Zhu J, Zou H, Liu Z. Mechanisms of cadmium-induced testicular injury: a risk to male fertility. Cells. 2022 Nov 14;11(22):3601.
- 9. Yan LJ, Allen DC. Cadmium-induced kidney injury: Oxidative damage as a unifying mechanism. Biomolecules. 2021 Oct 23;11(11):1575.
- 10. Paithankar JG, Saini S, Dwivedi S, Sharma A, Chowdhuri DK. Heavy metal associated health hazards: An interplay of oxidative stress and signal transduction. Chemosphere. 2021 Jan 1;262:128350.
- 11. Abarikwu SO, Wokoma AF, Mgbudom-Okah CJ, Omeodu SI, Ohanador R. Effect of Fe and Cd co-exposure on testicular steroid metabolism, morphometry, and spermatogenesis in mice. Biological Trace Element Research. 2019 Jul 15;190:109-23.
- 12. Soldin OP, O’Mara DM, Aschner M. Thyroid hormones and methylmercury toxicity. Biological trace element research. 2008 Dec;126:1-2.
- 13. Singh N, Kumar A, Gupta VK, Sharma B. Biochemical and molecular bases of lead induced toxicity in mammalian systems and possible mitigations. Chemical research in toxicology. 2018 Sep 4;31(10):1009-21.
- 14. Zheng Y, Zhang Q, Jing L, Fei Y, Zhao H. The effects of chronic lead exposure on testicular development of Japanese quail (Coturnix japonica): histopathological damages, oxidative stress, steroidogenesis disturbance, and hypothalamus-pituitary-testis axis disruption. Biological trace element research. 2023 Jul;201(7):3446-60.
- 15. Singh N, Kumar A, Gupta VK, Sharma B. Biochemical and molecular bases of leadinduced toxicity in mammalian systems and possible mitigations. Chemical research in toxicology. 2018 Sep 4;31(10):1009-21.
- 16. Zafar A, Javed S, Akram N, Naqvi SA. Health Risks of Mercury. InMercury Toxicity Mitigation: Sustainable Nexus Approach 2024 Feb 13 (pp. 67-92). Cham: Springer Nature Switzerland.
- 17. Aquino NB, Sevigny MB, Sabangan J, Louie MC. The role of cadmium and nickel in estrogen receptor signaling and breast cancer: metalloestrogens or not?. Journal of Environmental Science and Health, Part C. 2012 Jul 1;30(3):189-224.
- 18. Thévenod F. Cadmium and cellular signaling cascades: to be or not to be?. Toxicology and applied pharmacology. 2009 Aug 1;238(3):221 39.
- 19. de Souza ID, de Andrade AS, Dalmolin RJ. Lead-interacting proteins and their implication in lead poisoning. Critical reviews in toxicology. 2018 May 28;48(5):375-86.
- 20. Wisessaowapak C, Watcharasit P, Satayavivad J. Arsenic disrupts neuronal insulin signaling through increasing free PI3K-p85 and decreasing PI3K activity. Toxicology Letters. 2021 Oct 1;349:40-50.
- 21. Treas J, Roy P, Singh KP. Chronic coexposure to arsenic and estrogen potentiates genotoxic estrogen metabolic pathway and hypermethylation of DNA glycosylase MBD4 in human prostate epithelial cells. The Prostate. 2022 Sep;82(13):1273-83.
- 22. Khan F, Momtaz S, Abdollahi M. The relationship between mercury exposure and epigenetic alterations regarding human health, risk assessment and diagnostic strategies. Journal of Trace Elements in Medicine and Biology. 2019 Mar 1;52:37-47.
- 23. Stoica A, Katzenellenbogen BS, Martin MB. Activation of estrogen receptor-α by the heavy metal cadmium. Molecular Endocrinology. 2000 Apr 1;14(4):545-53.
- 24. Sabir S, Akash MS, Fiayyaz F, Saleem U, Mehmood MH, Rehman K. Role of cadmium and arsenic as endocrine disruptors in the metabolism of carbohydrates: Inserting the association into perspectives. Biomedicine & pharmacotherapy. 2019 Jun 1;114:108802.
- 25. Balali-Mood M, Naseri K, Tahergorabi Z, Khazdair MR, Sadeghi M. Toxic mechanisms of f ive heavy metals: mercury, lead, chromium, cadmium, and arsenic. Frontiers in pharmacology. 2021 Apr 13;12:643972.
- 26. Hanas JS, Larabee JL, Hocker JR. Zinc finger interactions with metals and other small molecules. Zinc finger proteins: from atomic contact to cellular function. 2005:39-46.
- 27. Benoff SH, Millan C, Hurley IR, Napolitano B, Marmar JL. Bilateral increased apoptosis and bilateral accumulation of cadmium in infertile men with left varicocele. Human Reproduction. 2004 Mar 1;19(3):616-27.
- 28. Akinloye O, Arowojolu AO, Shittu OB, Anetor JI. Cadmium toxicity: a possible cause of male infertility in Nigeria. Reprod Biol. 2006 Mar 1;6(1):17-30.
- 29. Schmitt CJ, Caldwell CA, Olsen B, Serdar D, Coffey M. Inhibition of erythrocyte δ-aminolevulinic acid dehydratase (ALAD) activity in fish from waters affected by lead smelters. Environmental monitoring and assessment. 2002 Jul;77:99-119.
- 30. Nazarinasab M, Behrouzian F, Abdi L, Moghaddam AA, Sadeghi S. Investigating the effect of magnesium supplement in patients with major depressive disorder under selective serotonin reuptake inhibitor treatment. Journal of Family Medicine and Primary Care. 2022 Dec 1;11(12):7800-5.
- 31. Ursinyova M, Uhnakova I, Serbin R, Masanova V, Husekova Z, Wsolova L. The relation between human exposure to mercury and thyroid hormone status. Biological trace element research. 2012 Sep;148:281-91.
- 32. Varoni MV, Palomba D, Macciotta NP, Antuofermo E, Deiana G, Baralla E, Anania V, Demontis MP. Brain renin-angiotensin system modifies the blood pressure response to intracerebroventricular cadmium in rats. Drug and chemical toxicology. 2010 Jul 1;33(3):302-9.
Data Sharing Statement
Funding
Author Contributions
Ethics Declaration
Acknowledgements
About this article
Cite this article
Saxena S, Saxena R, Singh AP. Endocrine disruption by heavy metals: an in-depth analytical review. RFP Jour of Bio and Biophy. 2025;10(2):51–60.
Licence:
Attribution-Non-commercial 4.0 International (CC BY-NC 4.0)This license enables reusers to distribute, remix, adapt, and build upon the material in any medium or format for noncommercial purposes only, and only so long as attribution is given to the creator.
| Received | Accepted | Published |
|---|---|---|
| August 04, 2025 | September 29, 2025 | December 24, 2025 |
DOI: 10.21088/rfpjbb.2582-3558.10225.2
Keywords
Endocrine DisruptionHeavy MetalsEnvironmental PollutantsLeadMercuryCadmiumArsenicSearch for Similar Articles
Similar Articles
- Pharmacological and Phytochemical Insights into Strobilanthes alternata: A Multi...
- The Unseen Pillars: The Foundational Role of Biophysics and Biochemistry in Mode...
- Screen time and its Biochemical Effect on Human beings
- How Alloying Affects the Critical Temperature of Low-Temperature Superconductors...
- Applications and Advancements of Molar Absorptivity in Clinical Biochemistry
Article Level Metrics
Last UpdatedMonday 26 January 2026, 16:10:58 (IST)
Accesses
Citations
Download citation
Article Keywords
Keyword Highlighting
Highlight selected keywords in the article text.
Timeline
| Received | August 04, 2025 |
| Accepted | September 29, 2025 |
| Published | December 24, 2025 |
licence
This license enables reusers to distribute, remix, adapt, and build upon the material in any medium or format for noncommercial purposes only, and only so long as attribution is given to the creator.