Original Article
Interesting Nuclear Magnetic Resonance studies of some N, N-bis(2-methoxyethyl) substituted Benzamides
Mamta Sharma, Sujeet Kumar Mewar
Licence:
Attribution-Non-commercial 4.0 International (CC BY-NC 4.0)This license enables reusers to distribute, remix, adapt, and build upon the material in any medium or format for noncommercial purposes only, and only so long as attribution is given to the creator
Red Flower's Journal of Forensic Chemistry and Toxicology 9(2):p 77-90, July-December 2023. | DOI: http://dx.doi.org/10.21088/jfct.2454.9363.9223.2
How Cite This Article:
Sharma M, Mewar SK. Interesting nuclear magnetic resonance studies of some N, N-bis(2-methoxyethyl) substituted benzamides. J Forensic Chem Toxicol. 2023;9(2):77–90.Timeline
Abstract
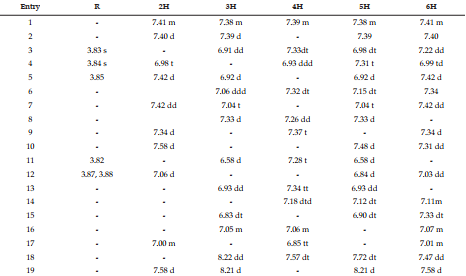
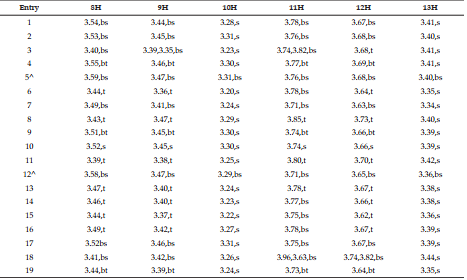
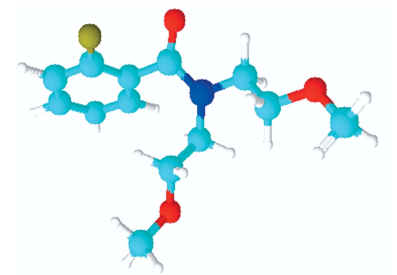
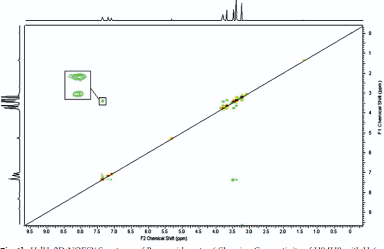
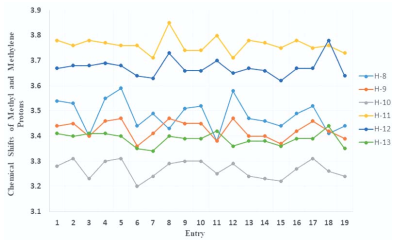
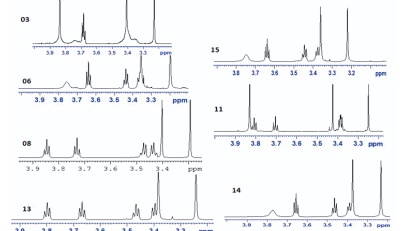
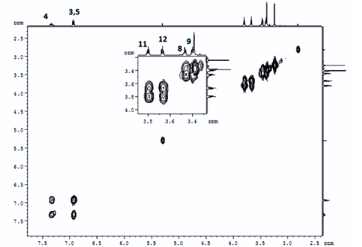
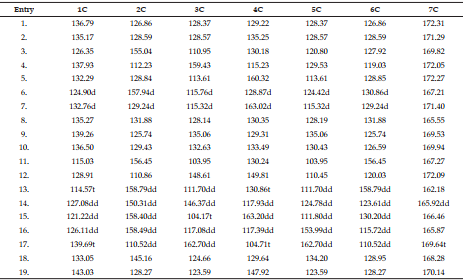
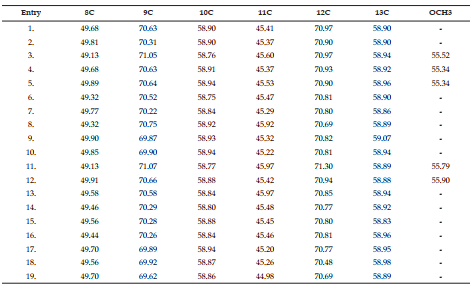
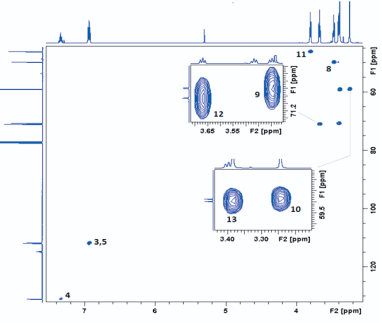
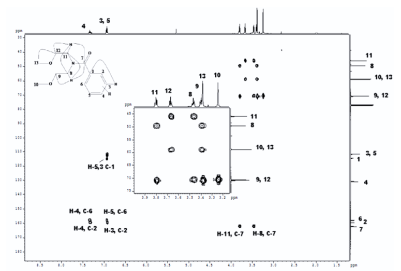
DEET (N, N Diethyl m-toluamide) and DEPA (Diethyl phenyl acetamide) are synthetic compounds and proven potent insecticide and repellent respectively. In the search of effective mosquito repellent, different derivatives of DEPA or substituted benzamides were synthesized and their NMR analysis was carried out at low temperature. Sterically Crowded Bis (2-methoxyethyl) substituted Benzamides possess low rotational barrier and are floppy at room temperature. Alkyl arms attached to nitrogen become magnetically nonequivalent even at low temperature. Di ortho Substitution in benzamides enhanced hindered internal rotation and resulted splitting in methylene proton signals. The effect of substitution in benzene ring, on the splitting of methylene proton NMR is very well explained and the complete NMR data of substituted benzamides is described in this study for reference purpose.
References
- 1. Peterson C and Coats J 2001, Insect Repellents-Past, present and future Pestic. Outlook 12 154.
- 2. Cavrini F, Gaibani P, Pierro A M, Rossini G, Landini M P and Sambri V J. 2009 Chikungunya: an emerging and spreading arthropod-borne viral disease Infect Dev. Ctrie.3 10 744.
- 3. Monath T P, 2001 Yellow fever: an update The Lancet-Infectious Diseases1 1 11.
- 4. Frank C, Höhle M, Stark K and Lawrence J 2013 More reasons to dread rain on Vacation? Dengue fever in 42 German and United Kingdom Madeira tourists during autumn 2012 Eurosurveillance 1814 14.
- 5. Prakash S, Srivastava C P, Kumar S, Pandey K S, Kaushik M P and Rao K M 1990 N,N-Diethyl Phenyl Acetamide -A New Repellent for Periplaneta americana (Dictyoptera: Blattidae), Blattella germanica and Supella longipalpa (Dictyoptera: Blattellidae) Journal of Medical Entomology 27 6 962.
- 6. KalyanasundaramM and Mathew N 2006 N,NDiethyl Phenylacetamide (DEPA): A Safe and Effective Repellent for Personal Protection Against Hematophagous Arthropods J. of Medical Entomology, 43 3 518.
- 7. Trigg J K 1996 Evaluation of a eucalyptus-based repellent against Anopheles spp. in Tanzania J. of the American Mosquito Control Association 12 2 243.
- 8. Braverman Y, Chizov-Ginzburg A and Mullens B A 1999 Mosquito repellent attracts Culicoides imicola (Diptera: Ceratopogonidae) Journal of Medical Entomology 36 1 1 13.
- 9. Osimitz T G and Murphy J V 1997 Neurological effects associated with use of the insect repellent N,N-diethyl-m-toluamide J. Toxicol. Clin. Toxicol. 35 435.
- 10. Montalbetti C A G N, and Falque V 2005 Amide bond formation and peptide coupling Tetrahedron 6110827.
- 11. Mack H-G and Oberhammer H 1997 Planarity of N,N-Dimethylacetamide, (CH3)2NC(O)CH3 J. Am. Chem. Soc.119 153567.
- 12. Troganis A N, Sicilia E, Barbarossou K, Gerothanassis, I P and Russo N 2005 Solvation Properties of N-Substituted Cis and Trans Amides Are Not Identical: Significant Enthalpy and Entropy Changes Are Revealed by the Use of variable Temperature 1H NMR in Aqueous and Chloroform Solutions and ab Initio Calculations, J. Phys. Chem. A 109 51 11878.
- 13. Wiberg K B, Rablen, P R, Rush, D J, Keith, T A 1995 Amides. 3. Experimental and Theoretical Studies of the Effect of the Medium on the Rotational Barriers for N,N- Dimethylformamide and N,NDimethylacetamide J. Am. Chem. Soc. 117 15 4261.
- 14. Gutowsky H S and Holm C H 1956 Rate Processes and Nuclear Magnetic Resonance Spectra. II. Hindered Internal Rotation of Amides Journal of Chemical Physics25 6 1228.
- 15. Mphahlele M J, Maluleka M M, Rhyman L, Ramasami P and Mampa R M 2017 Spectroscopic, DFT, and XRD Studies of Hydrogen Bonds in N-Unsubstituted 2-Aminobenzamides Molecules 22 83 1.
- 16. Licea R Q, Valladares J F C, Quintero A C, Padilla C R, Guerra R T, Flores R G and Waksman N 2002 NMR Detection of Isomers Arising from Restricted Rotation of the C-N Amide Bond of N-Formyl-otoluidine and N,N’-bis-Formyl-o-tolidine Molecules 7 8 662.
- 17. Rabinovitz M and Pines A 1969 Hindered internal rotation and dimerization of N,Ndimethylformamide in carbon tetrachloride J. Am. Chem. Soc.91 7 1585.
- 18. Umemoto K and Ouchi K Hindered internal rotation and intermolecular interactions 1985Proc. Indian Acad. Sci. (Chem. Sci.)94 11.
- 19. Pluth M D, Bergman R G and Raymond K N 2008 Acceleration of Amide Bond Rotation by Encapsulation in the Hydrophobic Interior of a Water-Soluble Supramolecular Assembly J. Org. Chem.73 18 7132.
- 20. Skorupska E A, Nazarski R B, Ciechanska M, Jozwiak A, K1ys A 2013 Dynamic 1H NMR spectroscopic study of hindered internal rotation in selected N,Ndialkyl isonicotinamides: an experimental and DFT analysis Tetrahedron 69 38 8147.
- 21. Gasparro F P, and Kolodny N H 1977 NMR determination of the rotational barrier in N,Ndimethylacetamide. A physical chemistry experiment J. of Chem. Edu54 4 258.
- 22. Wiberg K B and Rablen P R 1995 Why Does Thioformamide Have a Larger Rotational Barrier Than Formamide ? J. Am. Chem. Soc.117 8 2201.
- 23. Drakenberg T, Dahlqvist K I and Forsen S 1972 Barrier to internal rotation in amides. IV. N,NDimethylamides. Substituent and solvent effects The Journal of Physical Chemistry 76 15 2178.
- 24. Kaur D, Sharma P, Bharatam P V and Dogra N, 2006 Substituent and solvent effects on the rotational barriers in selenoamides: A theoretical studyJ. Mol. Struct.(Theochem)759 1-3 41.
- 25. Kang Y K and Park H S 2004 Internal rotation about the C–N bond of amides J. Mol. Struct. (Theochem)676 171.
- 26. Duffy E M, Severance D L, and Jorgensen W L 1992 Solvent effects on the barrier to isomerization for a tertiary amide from ab initio and Monte Carlo calculations J. Am. Chem. Soc.114 19 7535.
- 27. Gao J 1994 Origin of the solvent effects on the barrier to amide isomerization from the combined QM/MM Monte Carlo simulations Proc. Indian Acad. Sci. (Chem. Sci.)106 2 507.
- 28. Fong C W and Grant H G 1980 The effect of solvents on the 13C NMR chemical shifts of the carbonyl carbon and the rotational barriers of N,N dimethylbenzamide Organic Magnetic Resonance 14 2 147.
- 29. Fong C W and Grant H G 1981 Solvent Effects on the Carbon - 13 NMR Chemical Shifts and Rotational Barriers of N,N-Dimethylbenzamide-Solvent Enhancedπ Polarization Z. Naturforsch 36b 585.
- 30. Woodbrey J C and Rogers M T 1962 Solvent Effects on the Energy Barrier for Hindered Internal Rotation in Some N,N-Disubstituted Amides J. Am. Chem. Soc.84 1 13.
- 31. Hammaker R M and Gugler B A 1965 An NMR study of hindered internal rotation in N,N-dialkyl amides. Journal of Molecular Spectroscopy17 2 356.
- 32. Pinto B M, Szarek W A and Grindley T B 1984 Effects of substitution on nitrogen on barriers to rotation of amides. 2-Evaluation of the importance of resonance effects Magnetic Resonance in Chemistry, 22 11 676.
- 33. Siddall III T H and Garner R H, 1966 Some Studies of Slow rotation around bonds in amides Canadian Journal of Chem. 44 20 2387.
- 34. Dürst T, Gryff-Keller A and Terpiňski J 1983 Investigations on N,N-dialkylbenzamides by NMR spectroscopy: 5—Analysis of static and dynamic proton NMR spectra of 2-fluoro and 2,6-difluoroN,N-dimethyl and N,N-diethyl benzamides Org. Mag. Reso. 21 11 657.
- 35. Keller A G and Szczecinski P 1978 Remarks on the analysis of dynamic 1H NMR spectra of A3B2 C3D2 spin systems. Internal rotation in N,Ndiethylbenzamide Org. Mag. Reson. 11 5 258.
- 36. Gasparro F P and Kolodny N H 1977 NMR determination of the rotational barrier inN,Ndimethylacetamide. A physical chemistry experiment J. of Chem. Edu54 4 258.
- 37. Abraham R J, Byrne J J, Griffiths L and Perez M 2006 1H chemical shifts in NMR: Part 23, the effect of dimethyl sulphoxide versus chloroform solvent on 1H chemical shifts Magn. Reson. Chem. 44 5 491.
- 38. Reeves L W, Shaddick R C and Shaw K N, 1971 Nuclear Magnetic Resonance Studiesof Multisite Chemical Exchange. III. Hindered Rotation in Dimethylacetamide, Dimethyl Trifluoro-acetamide, and Dimethyl Benzamide Canadian Journal of Chemistry 49 22 3683.
- 39. Reeves L W and Shaw K N, 1971 Nuclear Magnetic Resonance Studies of Multi-siteChemical Exchange. II. Hindered Rotation in N, N-Dimethyl Carbamyl FluorideCanadian Journal of Chemistry49 22 3671.
- 40. Bowles P, Clayden J, Helliwell M, McCarthy C, Tomkinson M and Westlund N 1997Atroposelectivity in the reactions of ortholithiated aromatic tertiary amides with aldehydes J. Chem. Soc. Perkin Trans. 1 2607.
- 41. Clayden J, Foricher Y J Y and Lam H K 2002 Intermolecular Dearomatising Addition of Organolithium Compoundsto N-Benzoylamides of 2,2,6,6-Tetram ethylpiperidine. Eur. J. Chem. 3558.
- 42. Mujika J I, Gorostidi Doctoral Dissertation Dec 2005 Twisted amides: characterization of their electronic structure and analysis of their accelerated hydrolysis http://www.ehu.eus/chemistry/ theory/Files/tesis_joni.pdf.
- 43. Lewin A H 1964 The Question of Long/Range SpinSpin Coupling through Space: H-F Splitting over Six Bonds J. Am. Chem. Soc.86 11 2303.
- 44. Hu X, Zhang W, Carmichael I and Serianni A S 2010 Amide Cis−Trans Isomerizationin Aqueous Solutions of Methyl N-Formyl-d-glucosaminides and Methyl N-Acetyl-d-glucosaminides: Chemical Equilibria and Exchange Kinetics J. Am. Chem. Soc. 132 13 4641.
- 45. Abraham R J,Aboitiz N, Filippi M, Genesio E,Piaggio P and Sancassan F 2015Conformational analysis, part 43†. A theoretical and LIS/NMR investigation of the conformations of substituted benzamides Mag. Reson. in Chem.53 7 498.
- 46. Wiberg K B, Rablen P R, Rush D J and Keith T A 1995 Amides. 3. Experimental and Theoretical Studies of the Effect of the Medium on the Rotational Barriers for N, N-Dimethylformamide and N, N-Dimethylacetamide J. Am. Chem. Soc. 117 15 4261.
- 47. Du Ya, Hyster T D and Rovis T 2011 Rhodium (iii) catalyzed oxidative carbonylation of benzamides with carbon monoxide Chem. Commun.47 12074.
- 48. Li M,WangC, FangP and GeH 2011Pd(II)-catalyzed decarboxylative cross-coupling of oxamic acids with potassium phenyltrifluoroborates under mild conditions Chem. Commun.47 2011 6587.
- 49. Harikrishna K, Balasubramaniam S, Rakshit A & Aidhen I S 2015 Some interesting 1H NMR features of ortho substituted N-methoxy-N-methyl benzamides Ind. J. of Chem. Sec. B Org. and Med. Chem.54B 77.
- 50. Stevenson P J 2011 Second-order NMR spectra at high field of common organic functional groups Org. Biomol. Chem. 9 2078.
Data Sharing Statement
Funding
Author Contributions
Ethics Declaration
Acknowledgements
Conflicts of Interest
About this article
Cite this article
Sharma M, Mewar SK. Interesting nuclear magnetic resonance studies of some N, N-bis(2-methoxyethyl) substituted benzamides. J Forensic Chem Toxicol. 2023;9(2):77–90.
Licence:
Attribution-Non-commercial 4.0 International (CC BY-NC 4.0)This license enables reusers to distribute, remix, adapt, and build upon the material in any medium or format for noncommercial purposes only, and only so long as attribution is given to the creator
| Received | Accepted | Published |
|---|---|---|
| May 30, 2023 | June 30, 2023 | December 12, 2023 |
DOI: http://dx.doi.org/10.21088/jfct.2454.9363.9223.2
Keywords
Proton NMRCarbon NMRHeteronuclear Single Quantum Coherence Spectroscopy (HSQC)RotamersSearch for Similar Articles
Similar Articles
- H-NMR based Metabolic Fingerprinting in Forensic Investigations
- Sudden Death Due to 5-Fluorouracil-Induced Cardiotoxicity in a Case of Esophagea...
- Boerhaave Syndrome Masquerading as Sudden Death: A Virtual Autopsy Perspective
- Planned Complex Suicide Involving Phenyl Ingestion and Hanging: A Medico-Legal...
- A Fatal Journey of a Clot: Pulmonary Thromboembolism Case Report
Article Level Metrics
Last UpdatedMonday 26 January 2026, 20:48:44 (IST)
Accesses
Citations
Download citation
Article Keywords
Keyword Highlighting
Highlight selected keywords in the article text.
Timeline
| Received | May 30, 2023 |
| Accepted | June 30, 2023 |
| Published | December 12, 2023 |
licence
This license enables reusers to distribute, remix, adapt, and build upon the material in any medium or format for noncommercial purposes only, and only so long as attribution is given to the creator